Page 95 - Genetics_From_Genes_to_Genomes_6th_FULL_Part2
P. 95
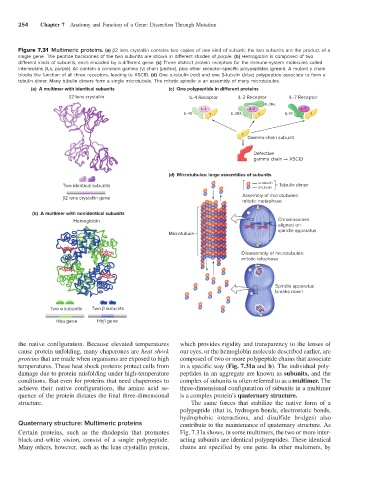
254 Chapter 7 Anatomy and Function of a Gene: Dissection Through Mutation
Figure 7.31 Multimeric proteins. (a) β2 lens crystallin contains two copies of one kind of subunit; the two subunits are the product of a
single gene. The peptide backbones of the two subunits are shown in different shades of purple. (b) Hemoglobin is composed of two
different kinds of subunits, each encoded by a different gene. (c) Three distinct protein receptors for the immune-system molecules called
interleukins (ILs; purple). All contain a common gamma (γ) chain (yellow), plus other receptor-specific polypeptides (green). A mutant γ chain
blocks the function of all three receptors, leading to XSCID. (d) One α-tubulin (red) and one β-tubulin (blue) polypeptide associate to form a
tubulin dimer. Many tubulin dimers form a single microtubule. The mitotic spindle is an assembly of many microtubules.
(a) A multimer with identical subunits (c) One polypeptide in di erent proteins
2 lens crystallin IL-4 Receptor IL-2 Receptor IL-7 Receptor
IL-2R
IL-4 IL-2 IL-7
IL-4R IL-2Rß IL-7R
Gamma-chain subunit
Defective
gamma chain XSCID
(d) Microtubules: large assemblies of subunits
-tubulin
Two identical subunits -tubulin Tubulin dimer
Assembly of microtubules:
2 lens crystallin gene
mitotic metaphase
(b) A multimer with nonidentical subunits
Hemoglobin Chromosomes
aligned on
spindle apparatus
Microtubule
Disassembly of microtubules:
mitotic telophase
Spindle apparatus
breaks down
Two subunits Two subunits
Hb gene Hb gene
the native configuration. Because elevated temperatures which provides rigidity and transparency to the lenses of
cause protein unfolding, many chaperones are heat shock our eyes, or the hemoglobin molecule described earlier, are
proteins that are made when organisms are exposed to high composed of two or more polypeptide chains that associate
temperatures. These heat shock proteins protect cells from in a specific way (Fig. 7.31a and b). The individual poly-
damage due to protein misfolding under high-temperature peptides in an aggregate are known as subunits, and the
conditions. But even for proteins that need chaperones to complex of subunits is often referred to as a multimer. The
achieve their native configurations, the amino acid se- three-dimensional configuration of subunits in a multimer
quence of the protein dictates the final three-dimensional is a complex protein’s quaternary structure.
structure. The same forces that stabilize the native form of a
polypeptide (that is, hydrogen bonds, electrostatic bonds,
hydrophobic interactions, and disulfide bridges) also
Quaternary structure: Multimeric proteins contribute to the maintenance of quaternary structure. As
Certain proteins, such as the rhodopsin that promotes Fig. 7.31a shows, in some multimers, the two or more inter-
black-and-white vision, consist of a single polypeptide. acting subunits are identical polypeptides. These identical
Many others, however, such as the lens crystallin protein, chains are specified by one gene. In other multimers, by