Page 62 - Genetics_From_Genes_to_Genomes_6th_FULL_Part3
P. 62
356 Chapter 10 Genome Annotation
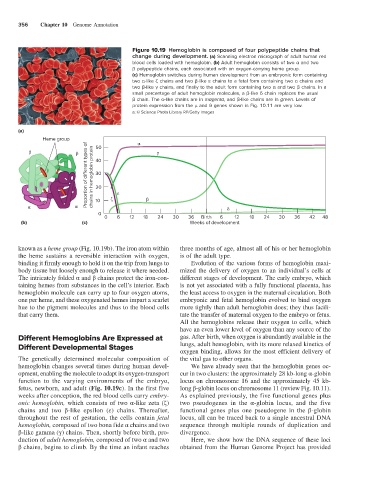
Figure 10.19 Hemoglobin is composed of four polypeptide chains that
change during development. (a) Scanning electron micrograph of adult human red
blood cells loaded with hemoglobin. (b) Adult hemoglobin consists of two α and two
β polypeptide chains, each associated with an oxygen-carrying heme group.
(c) Hemoglobin switches during human development from an embryonic form containing
two α-like ζ chains and two β-like ε chains to a fetal form containing two α chains and
two β-like γ chains, and finally to the adult form containing two α and two β chains. In a
small percentage of adult hemoglobin molecules, a β-like δ chain replaces the usual
β chain. The α-like chains are in magenta, and β-like chains are in green. Levels of
protein expression from the μ and θ genes shown in Fig. 10.11 are very low.
a: © Science Photo Library RF/Getty Images
(a)
Heme group
Proportion of di erent types of chains in hemoglobin protein 30
50
40
20
10
0
0 6 12 18 24 30 36 Birth 6 12 18 24 30 36 42 48
(b) (c) Weeks of development
known as a heme group (Fig. 10.19b). The iron atom within three months of age, almost all of his or her hemoglobin
the heme sustains a reversible interaction with oxygen, is of the adult type.
binding it firmly enough to hold it on the trip from lungs to Evolution of the various forms of hemoglobin maxi-
body tissue but loosely enough to release it where needed. mized the delivery of oxygen to an individual’s cells at
The intricately folded α and β chains protect the iron-con- different stages of development. The early embryo, which
taining hemes from substances in the cell’s interior. Each is not yet associated with a fully functional placenta, has
hemoglobin molecule can carry up to four oxygen atoms, the least access to oxygen in the maternal circulation. Both
one per heme, and these oxygenated hemes impart a scarlet embryonic and fetal hemoglobin evolved to bind oxygen
hue to the pigment molecules and thus to the blood cells more tightly than adult hemoglobin does; they thus facili-
that carry them. tate the transfer of maternal oxygen to the embryo or fetus.
All the hemoglobins release their oxygen to cells, which
have an even lower level of oxygen than any source of the
Different Hemoglobins Are Expressed at gas. After birth, when oxygen is abundantly available in the
Different Developmental Stages lungs, adult hemoglobin, with its more relaxed kinetics of
oxygen binding, allows for the most efficient delivery of
The genetically determined molecular composition of the vital gas to other organs.
hemoglobin changes several times during human devel- We have already seen that the hemoglobin genes oc-
opment, enabling the molecule to adapt its oxygen-transport cur in two clusters: the approximately 28 kb-long α-globin
function to the varying environments of the embryo, locus on chromosome 16 and the approximately 45 kb-
fetus, newborn, and adult (Fig. 10.19c). In the first five long β-globin locus on chromosome 11 (review Fig. 10.11).
weeks after conception, the red blood cells carry embry- As explained previously, the five functional genes plus
onic hemoglobin, which consists of two α-like zeta (ζ) two pseudogenes in the α-globin locus, and the five
chains and two β-like epsilon (ε) chains. Thereafter, functional genes plus one pseudogene in the β-globin
throughout the rest of gestation, the cells contain fetal locus, all can be traced back to a single ancestral DNA
hemoglobin, composed of two bona fide α chains and two sequence through multiple rounds of duplication and
β-like gamma (γ) chains. Then, shortly before birth, pro- divergence.
duction of adult hemoglobin, composed of two α and two Here, we show how the DNA sequence of these loci
β chains, begins to climb. By the time an infant reaches obtained from the Human Genome Project has provided